Almost all owners of a personal vehicle are well aware that there is acid in the batteries. Even beginners who are just beginning to comprehend the basics of driving, and even aware of this issue.
Many of them have heard about lead acid batteries, but in reality they have no idea how this device works. Meanwhile, certain chemical reactions take place here.
Content
What acid is in the battery and what is it for?
Most motorists are well aware of the acid in the battery. But there are those who believe that inside the battery there is nothing but distilled water (or distillate). Others are of the opinion in favor of hydrochloric acid, which is also incorrect.
Any car battery contains sulfuric acid - H2SO4. To be more precise, we are talking about a solution of sulfuric acid with distilled water. Such a liquid has a common name - electrolyte. So what is the role of sulfuric acid?
This is the main component for battery operation. In the absence of acid, the process of charging and discharging the battery is not possible. This is one of the most active species, which is able to interact with almost any metal, including their oxides. In addition, the acid can enter into metabolic reactions, and its activity depends on the water content.
When the acid battery charges, pure lead plates (negative) begin to release electrons that are taken up by lead oxide gratings (positive). When the battery is discharged, it happens exactly the opposite. In other words, when the plates give away the electrons, they are “destroyed” as it were — a charge occurs, and when discharged, they come back, which is called “restoration”.
And just for such a process of destruction - recovery, an aggressive environment is needed in the form of diluted sulfuric acid. And without it, the performance of car batteries would be at a very low level.
The composition of the electrolyte and how to do it right
Sulfuric acid is widely used in modern industry to produce electrical energy (batteries, batteries, electric capacitors). As for the composition of the electrolyte in the battery, the ratio between sulfuric acid and distilled water is as follows:
- acid itself - 30%;
- distilled water - 70%.
It is such a substance that effectively interacts with lead plates. In this case, the electrolyte density deserves special attention, which is directly influenced by sulfuric acid. In concentrated, it reaches an indicator of 1.83 g / cm3. By adding distilled water, the density is reduced to the desired limits - usually this is a range of 1.23-1.27 g / cm3.
| Density electrolyte (g / cm3) | Voltage without load (IN) | Voltage with load (IN) | Power charge (%) | Freezing electrolyte (FROM) |
|---|---|---|---|---|
| 1,27 | 12,66 | 10,8 | 100 | -60 |
| 1,26 | 12,6 | 10,66 | 94 | -55 |
| 1,25 | 12,54 | 10,5 | 87,5 | -50 |
| 1,24 | 12,48 | 10,34 | 81 | -46 |
| 1,23 | 12,42 | 10,2 | 75 | -42 |
| 1,22 | 12,36 | 10,06 | 69 | -37 |
| 1,21 | 12,3 | 9,9 | 62,5 | -32 |
| 1,2 | 12,24 | 9,74 | 56 | -27 |
| 1,19 | 12,18 | 9,6 | 50 | -24 |
| 1,18 | 12,12 | 9,46 | 44 | -18 |
| 1,17 | 12,06 | 9,3 | 37,5 | -16 |
| 1,16 | 12 | 9,14 | 31 | -14 |
| 1,15 | 11,94 | 9 | 25 | -13 |
| 1,14 | 11,88 | 8,84 | 19 | -11 |
| 1,13 | 11,82 | 8,68 | 12,56 | -9 |
| 1,12 | 11,76 | 8,54 | 6 | -8 |
| 1,11 | 11,7 | 8,4 | 0,0 | -7 |
To know this parameter is necessary to understand the freezing threshold of the electrolyte. At a density of 1.11 g / cm3 the substance freezes already under the influence of a relatively small cold: -7 ° C. For recommended values, this threshold is significantly different - from -58 ° C to -64 ° C. Is it possible to make an electrolyte yourself?
Yes, it is really possible, only it is necessary to act with utmost care. And since it is necessary to deal with sulfuric acid of high concentration, such work poses a certain danger. It is necessary to take care of the protection of the hands, body, respiratory organs.
Actually, there is nothing complicated in preparing the electrolyte for the battery yourself - mix sulfuric acid with distilled water, observing the proportion. It is worth noting that ordinary tap water is not suitable for such purposes, since it contains a large number of different impurities that adversely affect lead plates.
Actually the ingredients themselves:
- Sulfuric acid (density should be 1.83 g / cm3 or more, but not less).
- Distilled water.
- Any china.
The proportions of acid and water are known to us - 30% and 70%, respectively. At the same time, the nature of the approach to production is important - it is optimal to add acid to water, and not vice versa. It is also worth considering that when they are mixed a lot of thermal energy will be released and for this reason it is unacceptable to use glassware - it just bursts. When the temperature of the electrolyte drops, it can be poured into a glass container or container made of plastic.
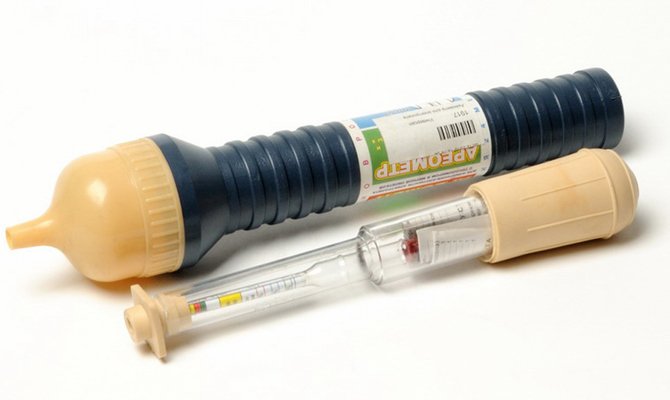
After the liquids are connected, the density should be measured with a hydrometer. If the indicators correspond to the permissible limit, the electrolyte is ready for operation. But such a device is far from every driver, and therefore the following tip for the density of the electrolyte (based on 1 liter of distilled water) is useful:
- at 1.23 g / cm3 - 280g;
- at 1.25g / cm3 - 310g;
- at 1.27g / cm3 - 345 g;
- at 1.29 g / cm3 - 385 g.
Actually, this is where the work ends. Those who live in the middle zone of Russia should adhere to a density of 1.27 g / cm3. Moreover, for areas with a cold climate (up to -30 ° C), the permissible indicator is 1.26-1.28 g / cm3, and hot subtropical regions - 1.24-1.26 g / cm3. Density limits from 1.27 g / cm3 up to 1.29 g / cm3 relevant for those regions where winter is rampant to -50 ° C.
What will lead to a breach
1.29 g / cm3 is not the highest - there is an electrolyte concentrate with a density of 1.33 g / cm3 (used for adjustment), previously could be found even with a density of 1.4 g / cm3but now it is out of sale. However, it should also be diluted with water and only then filled inside the battery. Why not pour highly concentrated electrolyte?
Nothing good will certainly happen! Due to the high concentration, the battery plates suffer - they simply corrode over time. This is slow but true! Therefore, if you pour a high concentrate, you should not be surprised that the battery soon failed.
Low electrolyte density leads to a phenomenon called sulfation. This process is known to many experienced drivers. As a result, lead sulfite crystals are deposited on the plates, due to which the metal loses its ability to accumulate charge.
In addition, as already mentioned above, due to too low density indicators, the electrolyte freezes, turning into ice. What this threatens, everyone already understands - damage to the plates cannot be avoided.
How to adjust fluid density
Car owners need to control the electrolyte level and its density. Due to the hydrolysis and heating of the batteries in the engine compartment, the content of the substance decreases, and the density increases on the contrary. For this reason, there is a need to add distilled water. But sometimes, the density of the electrolyte can become less than normal. Then you should increase the concentration of acid.
There are several ways to do this, based on the degree of decrease in electrolyte density. To do this, you should measure its concentration in each bank separately. If the electrolyte density is obtained from 1.18 g / cm3 up to 1.20 g / cm3, then the optimal solution is to replace a part of the electrolyte in the bank with a new one with a density of 1.27 g / cm3. In other words, an increase in the density of the electrolyte is made.
Only you should first make sure that the battery is charged, otherwise the battery should be recharged. With a low battery, this procedure cannot be started.Otherwise, the concentration of H2SO4 rises sharply, which will only lead to the destruction of the plates.
The procedure itself is performed in the following order:
- With a rubber bulb, pump out as much fluid as possible from the can. At the same time measure the volume.
- A new correction fluid with a density of 1.27-1.29 g / cm3 is added in an amount equal to half of the withdrawn volume.
- Let everything mix with each other - for this, you can give a load to the conclusions, just wait a while or shake the battery.
- Measure the density. If the indicators still have not reached the permissible limits, the electrolyte top-up should be continued until the desired parameters are reached.
- When the limit is set, the banks are closed, and the battery itself is put on charge.
In the case when the density of the electrolyte is reduced below the level of 1.2 g / cm3, then it is necessary to change it completely - merge the old one, fill in a new one.